Why You Need To Use FMO Controls For All Multicolor Flow Cytometry Experiments

What are the three most important parts of any flow cytometry experiment?
Controls, controls, controls.
Of course, this is mostly in jest. Mostly.
Reagent selection, well-maintained instruments, and other factors are also important. However, you must control for these factors.
Setting up the right controls is critical to determining how your cells are responding to treatment. It’s also important for correctly interpreting your data and drawing correct conclusions. Without the proper controls, you would not be able to compensate your flow cytometry experiments correctly or identify your cells of interest correctly.
By using the wrong controls, or leaving out certain controls altogether, you leave yourself wide open for criticism. Your gate placement will be questioned, your samples will be questioned, and your papers and grants will be questioned.
Why Fluorescence Minus One, Or FMO Controls, Are Important
We’ve all had experience working with someone who wanted to see the “real” samples, not the control samples, first.
But if you’re a smart scientist, you know “real,” or experimental samples are meaningless unless you know, among other things, the background levels you’re working with.
Valuing your control samples over your experimental samples is a mistake, especially when performing flow cytometry experiments. For example, in flow cytometry, some gates are “easy” to set. CD19+ B cells are often easy to pick out on a plot showing CD3 for a T cell subset sample. Other gates, however, are more difficult to define. This is especially true when you’re looking at activation markers within a continuum, or dim levels of positivity, like FoxP3 in regulatory T cells. How can you convince reviewers that you didn’t make an error and placed your gate in the proper place?
Now, consider gating out subsets in complicated 10+ color experiments. How are you going to account for the data spread that occurs with compensation? In any multicolor flow cytometry experiment, the answer to your gating troubles is to use Fluorescence Minus One, or FMO, controls.
What Is A Fluorescence Minus One, Or FMO, Control?
FMO controls are samples that contain all the antibodies you are testing in your experimental samples, minus one of them.
When analyzing the minus, or left out parameter in an FMO control, you give yourself a strong negative control to work with. It’s a strong negative control because the left out marker in the FMO control allows you to take into account how the other stains in your panel affect the left out parameter.
FMO controls are required for accurately discriminating positive versus negative signals, high versus low (or variable) antigen expression levels, and more. Even simple 2- or 3-color experiments reveal the need for FMO controls when drawing gates.
The figure below shows data from an experiment that was set up to identify naive versus memory CD4+ T cells. After properly compensating the data, the experimenter is left with the plot on the far right. Now the question becomes, where does the experimenter set the gate for PE positivity?
If the experimenter uses an unstained control only (far left panel), he or she is able to set a boundary (red dashed line) for PE positivity. However, if you now look at the middle plot, you can see that the bulk of the cells are labeled as “positive” for PE because they are above the boundary established by the unstained control.
Notice that the cells in the middle plot are stained with all the fluorochromes in the experimental samples except PE. As a result, they can NOT be positive for PE. This middle panel represents the FMO control in this experiment and shows the experimenter that the true negative boundary for PE, or FMO bound, is where the blue line lies.
Now, using the FMO control and resulting FMO bound, the experimenter is able to set the proper gate and ensure that the spread of the data (blue double arrow on the far right), is not impacting his or her gating.
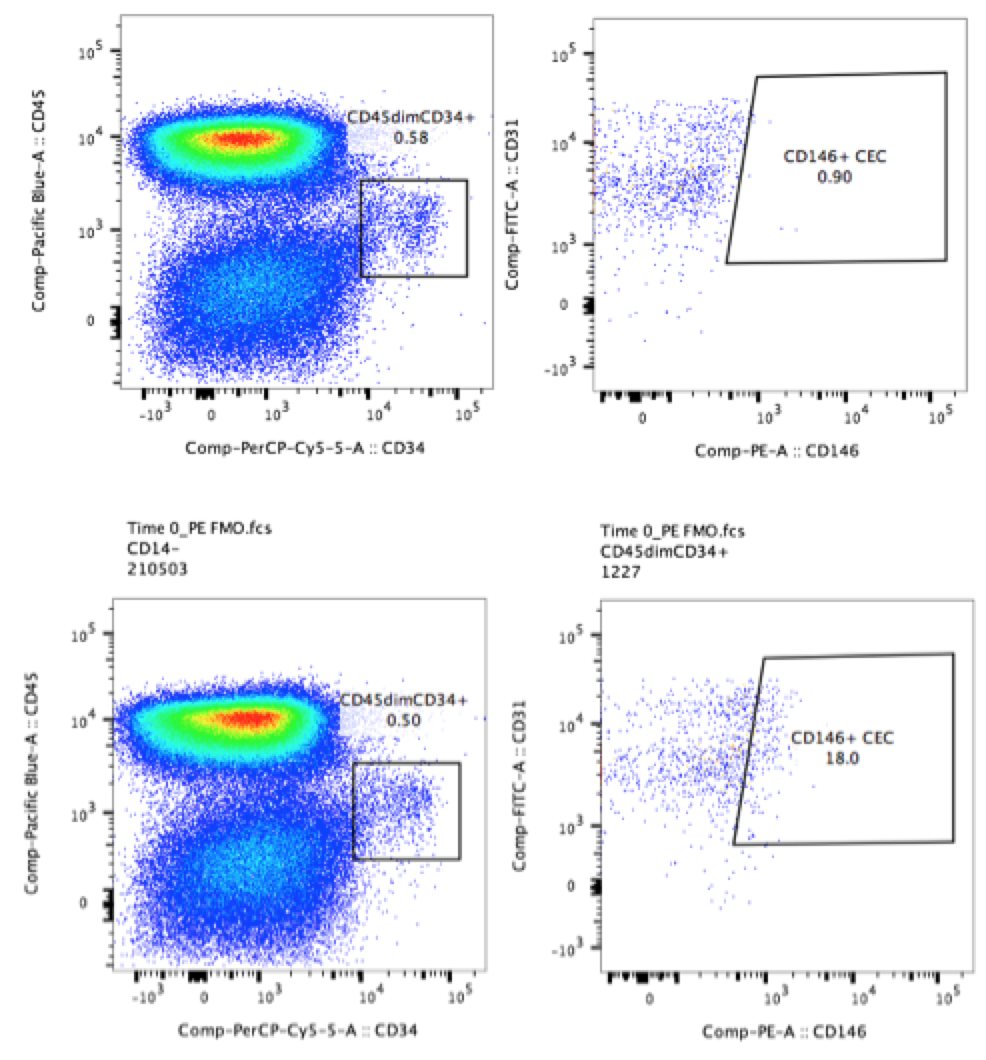
In the more complex example below, the plots show a population of circulating CD34+ cells in human PBMCs. The experimenter who ran these samples wanted to determine the levels of CD146 on these cells. The experimenter included FMO controls when testing the panel (CD146 on PE and CD31 on FITC, bottom two plots). As a result, the experimenter was easily able to draw gates around the populations of interest despite low levels of background positivity (top two plots).
When To Use Fluorescence Minus One, Or FMO Controls
- When running any multicolor flow cytometry experiment, there is a spread in the data that quickly becomes apparent when you compensate your data. Due to the physics of fluorescence and the exponential scales used to display flow cytometry data, this spread is unavoidable. However, using FMO controls in your flow cytometry experiments can remove all ambiguity from your compensated multicolor plots.
- When antigens are dim or variably expressed, the use of FMO controls is critical. This is especially true when analyzing cell activation markers on immune cells. (Don’t forget those unstimulated biological controls here either!)
- Do NOT add isotype antibodies to your FMO controls. These antibodies do NOT add any useful information to the FMO. If you are working with myeloid cells and think you need an isotype control, prepare it separately.
- If you are unsure of the levels of expression you are dealing with in your samples or the sensitivity of your antibodies or samples, use FMO controls. It’s better to prepare a few controls you may not need than lose money, time, publications or grant funding by being stingy.
When in doubt, follow Maecker’s advice reported in Cytometry, “When high-quality monoclonal antibody conjugates are used at appropriate concentrations, they tend to have relatively low background staining. As such, in experiments of >4 colors, the major source of background staining tends to be fluorescence spillover. Because of this, the use of FMO controls has become both popular and prudent.”
Fluorescence Minus One, or FMO controls are critical for any scientist who wants to back up his or her drawing of flow cytometry gates. When you use FMO controls, you establish scientific evidence as to why the gates you drew are drawn correctly. This is true for any multicolor flow cytometry experiment. FMO controls will give you, as well as those who view your work with a critical eye, confidence in the degree of accuracy of your measurements.
To learn more about FMO controls, and to get access to all of our advanced materials including 20 training videos, presentations, workbooks, and private group membership, get on the Flow Cytometry Mastery Class wait list.

ABOUT TIM BUSHNELL, PHD
Tim Bushnell holds a PhD in Biology from the Rensselaer Polytechnic Institute. He is a co-founder of—and didactic mind behind—ExCyte, the world’s leading flow cytometry training company, which organization boasts a veritable library of in-the-lab resources on sequencing, microscopy, and related topics in the life sciences.
More Written by Tim Bushnell, PhD